Arsenic is the most important factor in the intractable gold ore. The content of arsenic in the earth's crust is about 0.0002% (2ppm). It is mainly in the form of sulfide or oxidized ore in nature. The main related to biooxidation is arsenopyrite. There are four oxidation states of arsenic: +3, +5, -3, 0, but there are only two oxidation states of As3+ and As5+ in aqueous solution. The arsenic released during the biooxidation process is mainly in the form of arsenate and arsenite in the oxidation state of As3+ and As5+. Therefore, arsenopyrite, As3+ and As5+ are the focus of this section. Arsenic arsenic minerals in refractory gold ores is generally arsenopyrite, their close sulfide minerals are mainly pyrite, pyrrhotite. In the biooxidation process, the three minerals are all aerobic. Among them, the oxidation process of pyrite is the only aerobic and acidogenic reaction. The oxidation of arsenopyrite and pyrrhotite is aerobic and acid-reactive. The reaction formula is as follows: The order and rate of oxidation of these three major sulfide minerals by bacteria during biooxidation are different. The order of oxidation by bacteria is firstly pyrrhotite, followed by arsenopyrite, and finally pyrite. Therefore, in the process of biooxidation, the proportion of various minerals in the feed directly affects the stability of the biooxidation process and the direction and oxidation rate of the reaction. If the sulfur content in the feed is too low, sulfuric acid needs to be added to increase the acidity of the solution; if the sulfur content is too high, limestone or lime is added to reduce the acidity. A more important reason is that arsenic has two forms of As3+10 and As5+ in solution, and the effect of each form on the biological oxidation process is very different. Studies have shown that when the content of poisonous sand and pyrrhotite in the feedstock of biooxidation pretreatment increases, the oxidation-reduction potential (melon) of the oxidation process decreases, and the concentration of Ag3+ in the solution increases. Studies have shown that pyrite is the main factor determining the oxidation state of arsenic during biooxidation. RM Nyashanu et al. tested arsenic-bearing ore samples from five mines in four countries. The results show that As'-'- plays a dominant role in the whole oxidation process of concentrates with high arsenic content. While other concentrates only dominate As3+ in the initial stage of the oxidation process. When there is more pyrite in the initial stage, it is rapidly oxidized to As5+, which indicates that as the solution can continuously replenish enough Fe2+ in the process of bacterial oxidation, the As3+ can be quickly oxidized to As5+. . Therefore, the ratio of various mineral components in the feed of the biological oxidation process plays an important role in the pH value of the oxidation process and the oxidation state of the arsenic.
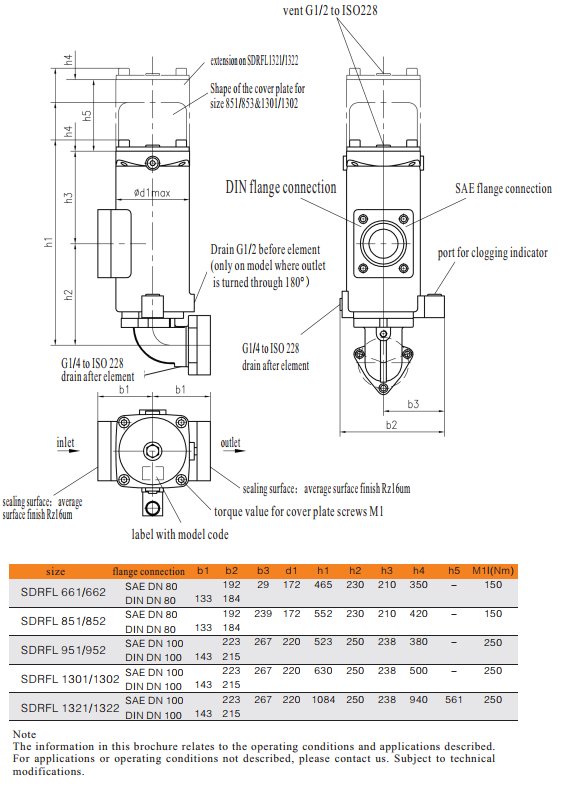
The SDRFL Cast Version Inline Hydraulic Filter is designed according to international regulations. It consists of a two piece filter housing with a bolt-on cover plate. Inlet and outlet positioned at the same side or opposite side.
BN/HC: 25 bar P/HC: 10 bar
Crane Filter,Machine Filter,Cast Version Filter,Stainless Steel Cast Version Filter Xinxiang Shengda Filtration Technique Co., Ltd. , https://www.shengdafiltration.com
Filter Element
Filter elements are validated according to ISO test standard, with the following pressure stability values:
Wire mesh( W/HC ): 30 bar V: 30 bar
Compatibility
It can be used for mineral oils, lubrication oils, non-flame fluids, synthetic and rapidly biodegradable fluids.
For water or other application, please contact us.